|
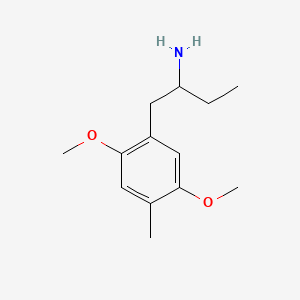
Name: (Positional Isomer: 1-(2,5-dimethoxy-4-methylphenyl)butan-2-amine) 2,5-Dimethoxyamphetamine
Type:
AKA: DMA, 2, 5-DMA

|
|
II. Natural Derivative
Synthetic substance, no natural derivative
 |
|
III. Chemical Profile (IUPAC name)
|
|
IV. History
In 1966, a chemist in Mexico, Dr. Luis Alvarado, was searching for new chemical compounds. He discovered a new type of amphetamine, 2,5 dimethoxyamphetamine (2,5 dimethoxyphenethylamine). This new drug was created by substituting the 3,4-methylenedioxy group with an amine group.
The 2,5 dimethoxy group is similar to the 3,4-dimethoxy group, so 2,5 dimethoxyamphetamine was considered to be the same drug. However, it is different from 3,4-dimethoxyamphetamine by the absence of the 3-hydroxy group.
This chemical compound was discovered in the first half of 1966, and was patented in 1966.
2,5 dimethoxyamphetamine was patented as a chemical compound. The chemical compound is named 2,5 dimethoxyamphetamine (2,5 dimethoxyphenethylam

|
|
V. Legal Information
This positional isomer of 2,5-dimethoxyamphetamine (2,5-DMA) is a synthetic psychedelic. Its legal status varies; in the United States, it is generally regulated under the Federal Analogue Act if intended for human consumption. Other countries also have regulations to manage its potential for abuse. [Source: UNODC].
US Federal Schedule - I
Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote.
Key US Federal Policies:
Controlled Substances Act. Public Law: Public Law 91-513 (text can be found on GovInfo) (https://www.dea.gov/drug-information/csa). Date enacted: October 27, 1970.
|
|
VI. Physical Effects
The positional isomer 1-(2,5-dimethoxy-4-methylphenyl)butan-2-amine, also known as 2,5-dimethoxyamphetamine, is a psychoactive compound with hallucinogenic effects. It alters perception and can induce euphoria. Short-term use may result in altered sensory experiences, but long-term use poses risks of psychological issues. Overdose risks include severe agitation and hallucinations. Safe use requires cautious dosing and monitoring. Recent research explores its hallucinogenic properties and associated health risks.  |
|
VII. Psychological Effects
This compound, a psychedelic, affects serotonin receptors, leading to altered perception and mood enhancement. Immediate effects include euphoria and sensory distortions, lasting several hours. Long-term use may result in persistent psychological effects and altered cognition. Research emphasizes its potency and potential for long-term psychological effects with chronic use.
 |
|
VIII. Culture
2,5-Dimethoxyamphetamine (DMA) is a psychedelic amphetamine producing hallucinogenic and stimulant effects, classifying it as an upper. Short-term use can enhance sensory perception and induce euphoria, while long-term use poses risks of psychological dependence and neurotoxicity. Overdose can result in severe agitation, hyperthermia, and cardiovascular complications. Safe dosages range from 10-20 mg. Recent studies explore its potential therapeutic uses and abuse risks. Physical signs include dilated pupils, increased heart rate, and elevated blood pressure.
 |