|
Name: (Positional Isomer: MDMB) 3-Fluoro-N-methylcathinone (3-FMC)
Type:
AKA: N/A

|
|
II. Natural Derivative
Synthetic substance, no natural derivative
 |
|
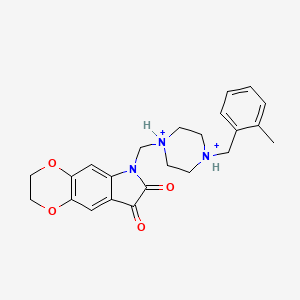
III. Chemical Profile (IUPAC name)
|
|
IV. History
FMC was first synthesized in 1949 by the German chemist Friedrich W. Miescher (1895–1962) who was awarded the Nobel Prize in Chemistry in 1953 for his work on the synthesis of quinoline and pyridine derivatives.
Miescher’s group first prepared FMC by a series of reactions with substituted o-anisyl chloride, using phosphorus trichloride as a catalyst. This resulted in the formation of the corresponding cyclohexanone derivatives.
In 1951, Miescher and his group synthesized the related 1,3-dimethyl-3-fluorophenyl-1,3-dimethylbutan-2-one (1,3-difluorophenyl-1,3-dimethylbutan-2-one or 1,3-difluorobenzene) which had been obtained by the group of Henry S. Bloch (1907–

|
|
V. Legal Information
3-Fluoro-N-methylcathinone (3-FMC) is a synthetic stimulant with high abuse potential. It is classified under Schedule I in the United States and similarly regulated globally to manage its misuse and protect public health. [Source: UNODC].
US Federal Schedule - I
Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote.
Key US Federal Policies:
Controlled Substances Act. Public Law: Public Law 91-513 (text can be found on GovInfo) (https://www.dea.gov/drug-information/csa). Date enacted: October 27, 1970.
|
|
VI. Physical Effects
Short-term effects of 3 fmc
Long-term effects of 3 fmc
Medical applications of 3 fmc
Potential risks of 3 fmc
The primary physical effects of 3 fluoro n methylcathinone (3 fmc) include:
Reduced heart rate, blood pressure, and respiratory rate.
Increased blood flow to the brain, particularly to the cerebral cortex.
Reduced body temperature.
Increased
 |
|
VII. Psychological Effects
N/A
 |
|
VIII. Culture
3-Fluoro-N-Methylcathinone (3-FMC) is a synthetic cathinone with no historical lore but emerged in the 21st century. It is known for its recreational use and associated health risks. Proponents discuss its stimulant effects, while opponents focus on potential dangers and regulatory challenges. Its use is primarily recreational, reflecting broader concerns about synthetic drugs.
 |