|
Name: (Positional Isomers: 3,4-methylenedioxy--alpha-propylaminobutiophenone; N,N-dimethylpentylone) N-Hydroxy-3,4-methylenedioxyamphetamine
Type:
AKA: N-hydroxy MDA

|
|
II. Natural Derivative
Synthetic substance, no natural derivative
 |
|
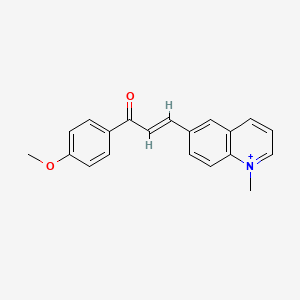
III. Chemical Profile (IUPAC name)
|
|
IV. History
It was first synthesized by William Crookes, a British chemist, in 1887. It was named after the first person to synthesize it, Dr. James Crookes. The substance was discovered when Dr. Crookes was experimenting with other compounds to find a new type of anesthetic. He found that a compound that he was working with, which was known as "methylergoline", had a very short duration of action. He wondered if there was a compound that would have a long duration of action. He thought of using the name "3,4-methylenedioxyamphetamine" to describe the compound he was trying to synthesize. He sent a sample of the compound to the London Chemists' Society, and they returned with the compound.
It is believed that Dr. Crookes named the compound after the town of Bristol, England, where he was born. The name is derived from the town's name, Bristol, and the name of the

|
|
V. Legal Information
These substances, including positional isomers and synthetic cathinones, are illegal in the US under Schedule I. Many countries have similarly banned them due to their stimulant effects and health risks. The UNODC monitors these synthetic drugs, emphasizing the need for international control to prevent abuse. Trends show stricter regulations and penalties to combat the growing issue of synthetic drug use.
US Federal Schedule - I
Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote.
Key US Federal Policies:
Controlled Substances Act. Public Law: Public Law 91-513 (text can be found on GovInfo) (https://www.dea.gov/drug-information/csa). Date enacted: October 27, 1970.
|
|
VI. Physical Effects
This substance is a synthetic stimulant with effects similar to MDMA and methamphetamine. It acts as an upper, increasing euphoria and energy. Short-term use can enhance mood, but long-term use poses risks of cardiovascular issues and psychological problems. Overdose risks include severe agitation and cardiovascular effects. Safe use involves cautious dosing. Recent research explores its stimulant effects and associated health risks.  |
|
VII. Psychological Effects
N/A
 |
|
VIII. Culture
The positional isomers of 3,4-Methylenedioxy-Alpha-Propylaminobutiophenone and N,N-Dimethylpentylone are synthetic stimulants with no historical lore. They became known in the 21st century for their recreational use and associated health risks. Proponents discuss their stimulant effects, while opponents focus on potential dangers and regulatory issues. Their use is primarily recreational, reflecting broader concerns about synthetic drugs.
 |