|
Name: 1-dimethylamino-1,2-diphenylethane, Lefetamine SR-18 (1-Cyclohexylethyl-3-(2-methoxyphenylacetyl) indole)
Type:
AKA: SR-18 and RCS-8

|
|
II. Natural Derivative
Synthetic substance, no natural derivative
 |
|
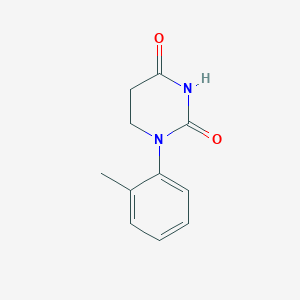
III. Chemical Profile (IUPAC name)
|
|
IV. History
1-Dimethylamino-1,2-diphenylethane, also known as Lefetamine, was first synthesized in the 1970s. It was developed as a stimulant and psychoactive compound with potential therapeutic uses. The compound's introduction reflects ongoing research into stimulants and their effects. Lefetamine's use in recreational settings has led to increased regulatory attention.

|
|
V. Legal Information
1-Dimethylamino-1,2-diphenylethane (Lefetamine SR-18) is a psychoactive compound with stimulant properties. It is controlled due to its potential for abuse and impact on mental health. [Source: UNODC].
US Federal Schedule - I
Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote.
Key US Federal Policies:
Controlled Substances Act. Public Law: Public Law 91-513 (text can be found on GovInfo) (https://www.dea.gov/drug-information/csa). Date enacted: October 27, 1970.
|
|
VI. Physical Effects
1-Dimethylamino-1,2-diphenylethane and Lefetamine are psychoactive compounds with stimulant and psychoactive effects. They act as uppers, enhancing alertness and mood. Short-term use can lead to euphoria and increased energy, but long-term use may pose risks of psychological effects and dependency. Overdose risks include severe agitation and cardiovascular issues. Safe use involves cautious dosing. Recent research focuses on their effects and health risks.  |
|
VII. Psychological Effects
Lefetamine affects dopamine and serotonin systems, causing euphoria and altered cognition. Immediate effects include mood enhancement and cognitive stimulation, lasting several hours. Long-term use can lead to dependence, anxiety, and cognitive impairments. Recent research indicates risks of significant mental health disturbances with chronic use, including severe mood swings and potential for addiction.
 |
|
VIII. Culture
This substance combines aspects of stimulants and psychoactives. Its cultural significance is related to its use in recreational settings and its complex effects. Media coverage may focus on the risks and effects associated with such substances. The substance is used recreationally rather than medicinally, contributing to discussions about drug regulation and harm reduction.
 |