|
Name: Brexanolone (3-alpha-hydroxy-5-alpha-pregnan-20-one)
Type: Neurosteroid
AKA: Allopregnanolone

|
|
II. Natural Derivative
Synthetic substance, no natural derivative
 |
|
III. Chemical Profile (IUPAC name)
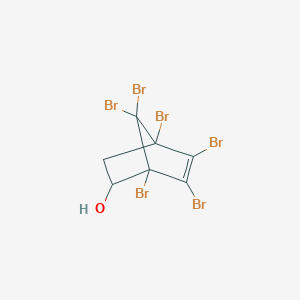
|
|
IV. History
Brexanolone, a synthetic analog of allopregnanolone, was approved by the FDA in 2019 for the treatment of postpartum depression. Its development represents a significant advancement in the treatment of mood disorders, offering a novel mechanism of action by modulating GABA receptors. Brexanolone has provided a new option for patients with severe postpartum depression.

|
|
V. Legal Information
Brexanolone, used for treating postpartum depression, is not classified as a controlled substance. It is approved for medical use with regulations focusing on its prescription and use in treating specific conditions rather than its control as a drug of abuse.
US Federal Schedule - IV
Schedule IV drugs, substances, or chemicals are defined as drugs with a low potential for abuse and low risk of dependence. Some examples of Schedule IV drugs are: Xanax, Soma, Darvon, Darvocet, Valium, Ativan, Talwin, Ambien, Tramadol.
Key US Federal Policies:
Controlled Substances Act. Public Law: Public Law 91-513 (text can be found on GovInfo) (https://www.dea.gov/drug-information/csa). Date enacted: October 27, 1970.
|
|
VI. Physical Effects
Brexanolone, a neurosteroid, is used for treating postpartum depression. As a downer, it induces sedation and mood stabilization. Short-term effects include improved mood and reduced depressive symptoms, while long-term use is generally safe with few risks. Overdose risks are minimal but may include sedation. Safe dosing involves medical supervision. Recent research highlights its effectiveness in treating postpartum depression with a need for further studies.  |
|
VII. Psychological Effects
Brexanolone, a neurosteroid, affects GABA-A receptors, providing rapid antidepressant effects. Immediate effects include mood improvement, with long-term use potentially offering benefits for depression. Research focuses on its efficacy for postpartum depression and overall mental health impact.
 |
|
VIII. Culture
Brexanolone, marketed as Zulresso, is used for postpartum depression (PPD). Its approval in 2019 marked a significant advancement in mental health treatment, especially for new mothers. Historically, PPD has been underrecognized and undertreated, with cultural stigmas surrounding maternal mental health. Brexanolone's introduction has opened up conversations about the importance of mental health care for women and the need for specialized treatments. In modern culture, it represents progress in addressing mental health with tailored therapies. Advocacy groups and healthcare providers highlight its benefits, while debates continue about access, cost, and broader mental health support.
 |