|
Name: Glutethimide
Type: Sedative
AKA: Doriden, Dorimide
|
|
II. Natural Derivative
Synthetic substance, no natural derivative
 |
|
III. Chemical Profile (IUPAC name)
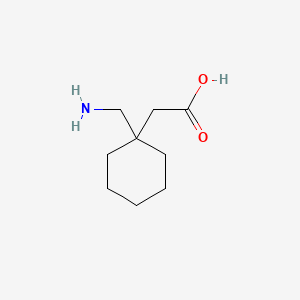
|
|
IV. History
Glutethimide is a hypnotic and sedative drug that was first introduced in the 1950s as a treatment for insomnia. It was marketed under the brand name Doriden. Glutethimide acts as a central nervous system depressant, similar to barbiturates. Due to its potential for abuse and dependence, glutethimide's use declined over the years, and it has been largely replaced by safer alternatives like benzodiazepines.

|
|
V. Legal Information
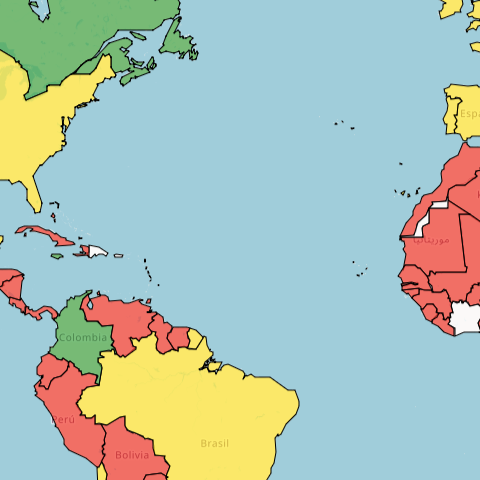
Glutethimide is a sedative-hypnotic drug that is classified as a Schedule II controlled substance in the US due to its potential for abuse. Globally, it is regulated under similar controls. Historically, its use has been limited due to concerns about addiction and overdose, with increasing restrictions reflecting the trend towards tighter controls on sedative drugs.
US Federal Schedule - II
Schedule II drugs, substances, or chemicals are defined as drugs with a high potential for abuse, with use potentially leading to severe psychological or physical dependence. These drugs are also considered dangerous. Some examples of Schedule II drugs are: combination products with less than 15 milligrams of hydrocodone per dosage unit (Vicodin), cocaine, methamphetamine, methadone, hydromorphone (Dilaudid), meperidine (Demerol), oxycodone (OxyContin), fentanyl, Dexedrine, Adderall, and Ritalin.
Key US Federal Policies:
Controlled Substances Act. Public Law: Public Law 91-513 (text can be found on GovInfo) (https://www.dea.gov/drug-information/csa). Date enacted: October 27, 1970.
|
|
VI. Physical Effects
Glutethimide, a sedative-hypnotic, is used for its calming effects. As a downer, it induces sedation and muscle relaxation. Short-term effects include drowsiness and reduced anxiety, while long-term use can lead to dependence and cognitive issues. Overdose risks include severe sedation, respiratory depression, and potentially death. Safe use involves adhering to prescribed doses, typically 250-500 mg. Recent research highlights its use in treating insomnia but also notes the risks of abuse and potential dependence.  |
|
VII. Psychological Effects
Glutethimide, a sedative-hypnotic, impacts GABA-A receptors, leading to reduced anxiety and sedation. Psychological effects include impaired cognition and mood changes. Long-term use can result in dependence and significant cognitive issues. Research focuses on its historical use, abuse potential, and impact on mental health.
 |
|
VIII. Culture
Glutethimide, a sedative-hypnotic drug introduced in the mid-20th century, was initially used as an alternative to barbiturates for treating insomnia. Its cultural significance lies in its representation of the shifting trends in pharmacotherapy for sleep disorders. However, like many sedatives, glutethimide’s potential for abuse and addiction became apparent, leading to its decline in medical use. The substance is part of the broader narrative of the search for effective and safe treatments for insomnia and anxiety. Glutethimide’s legacy is a cautionary tale of the unintended consequences of sedative use and the ongoing challenges in managing sleep and anxiety disorders.
 |