|
Name: ortho-Fluoroacryl fentanyl (N-(2-fluorophenyl)-N-(1-phenethylpiperidin-4-yl)acrylamide)
Type: Synthetic opioid
AKA: N/A

|
|
II. Natural Derivative
Synthetic substance, no natural derivative
 |
|
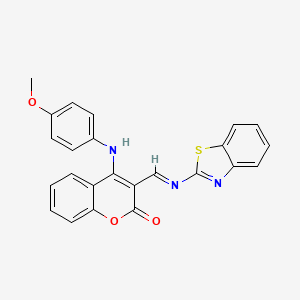
III. Chemical Profile (IUPAC name)
|
|
IV. History
In December 2002, researchers from the University of North Carolina, Duke University and the University of Florida discovered a substance called the "cocaine derivative" that had the potential to become a powerful painkiller. In their research, the researchers discovered that the substance acted as an opiate and was similar to the opium poppy plant. The substance was nicknamed "ortho fluoroacryl fentanyl" and was created by researchers at the University of North Carolina, Duke University and the University of Florida.
In 2004, the drug was patented by the University of North Carolina, Duke University and the University of Florida and then licensed to a company called K-Line Pharmaceuticals, Inc. The drug was then developed into a prescription drug called oxycodone. Oxycodone was made by a company called Purdue Pharma, which was owned by the drug company Purdue Pharma, Inc. The drug was used to treat moderate to severe pain in patients suffering from moderate to severe pain.
On October

|
|
V. Legal Information
Ortho-Fluoroacryl Fentanyl, a potent synthetic opioid, is classified as a Schedule I substance in the US, making it illegal. Its high potential for abuse and overdose lead to similar bans in many countries. The UNODC and other regulatory bodies monitor synthetic opioids, emphasizing strict control measures to prevent misuse and address the opioid crisis.
US Federal Schedule - I
Schedule I drugs, substances, or chemicals are defined as drugs with no currently accepted medical use and a high potential for abuse. Some examples of Schedule I drugs are: heroin, lysergic acid diethylamide (LSD), marijuana (cannabis), 3,4-methylenedioxymethamphetamine (ecstasy), methaqualone, and peyote.
Key US Federal Policies:
Controlled Substances Act. Public Law: Public Law 91-513 (text can be found on GovInfo) (https://www.dea.gov/drug-information/csa). Date enacted: October 27, 1970.
|
|
VI. Physical Effects
Ortho-fluoroacryl fentanyl is a synthetic opioid analog with strong analgesic properties. It acts as a downer, causing sedation, severe respiratory depression, and constricted pupils. Short-term use provides effective pain relief, while long-term use poses risks of addiction, tolerance, and significant health issues. Overdose can result in fatal respiratory depression. Safe use demands precise dosing and medical supervision. Recent research underscores its potency and overdose risks.  |
|
VII. Psychological Effects
Ortho-fluoroacryl fentanyl, an opioid, affects opioid receptors, causing euphoria and cognitive impairment. Immediate effects include mood enhancement and pain relief, lasting several hours. Long-term use can lead to dependence and psychological issues such as depression. Research indicates significant mental health risks with chronic use, including severe mood disturbances and potential for addiction.
 |
|
VIII. Culture
Ortho-fluoroacryl fentanyl is a synthetic opioid, classifying it as a downer. Short-term use provides potent analgesic effects, while long-term use can lead to dependence, tolerance, and severe health risks. Overdose risks are extremely high, leading to severe respiratory depression and potentially fatal outcomes. Safe dosages are not well-established, with use often limited to controlled medical environments. Recent research emphasizes its potency and associated health risks. Physical effects include drowsiness, constricted pupils, and respiratory depression.
 |